Ready to conquer the intricate world of organic chemistry nomenclature? Understanding Naming Hydrocarbons (Alkanes, Alkenes, Alkynes) isn't just about memorizing rules; it's about speaking the universal language of chemistry. Whether you're a student grappling with your first organic course, a researcher ensuring precision, or simply curious about the building blocks of life, mastering the International Union of Pure and Applied Chemistry (IUPAC) system for hydrocarbons is a fundamental skill.
This isn't your average textbook chapter. We're going to demystify the process, breaking down the IUPAC rules into manageable, human-readable steps. Think of this as your practical playbook for confidently assigning or deciphering the name of any straight-chain or branched alkane, alkene, or alkyne you encounter.
At a Glance: Your Hydrocarbon Naming Playbook
- Alkanes: Saturated hydrocarbons with only single C-C bonds. Naming involves finding the longest chain, numbering substituents for the lowest possible numbers, and listing them alphabetically.
- Alkenes: Unsaturated hydrocarbons with at least one C=C double bond. The parent chain must contain the double bond, and numbering prioritizes the double bond's position. Suffix changes to "-ene."
- Alkynes: Unsaturated hydrocarbons with at least one C≡C triple bond. Similar rules to alkenes, but the suffix changes to "-yne."
- Multiple Bonds: Compounds with multiple double bonds use "-diene," "-triene," etc. For mixed double and triple bonds, the double bond gets naming preference (e.g., -en-yne).
- Geometric Isomers: Alkenes can exhibit 'cis/trans' or 'E/Z' isomerism due to restricted rotation around the double bond. These designations are critical for distinguishing unique molecules.
- Cyclic Variants: For hydrocarbons forming rings, a "cyclo-" prefix is added to the parent name.
- The Golden Rule: Always prioritize the multiple bond's position for alkenes and alkynes when numbering the parent chain.
Why Bother with a Systematic Naming System?
Imagine a world where every compound had a whimsical nickname. "Oil of vitriol" sounds poetic, but how precise is it when you're ordering concentrated sulfuric acid for a delicate reaction? Early chemists often named compounds based on their source (like "formic acid" from ants, formica) or their properties. This was fine for a handful of known substances, but as our understanding of molecular structure exploded, so did the potential for confusion.
Enter IUPAC. This global authority stepped in to standardize chemical nomenclature, creating a set of logical, unambiguous rules. The IUPAC system ensures that every unique chemical structure has one unique name, and every unique name corresponds to one unique structure. This universal language prevents errors in research, manufacturing, and even medicine, where the precise identity of a molecule can be a matter of life or death.
For hydrocarbons—the fundamental building blocks of organic chemistry, made solely of carbon and hydrogen—this systematic approach is essential. They might seem simple, but their structural diversity, from straight chains to complex branches and rings, demands clarity.
The Foundation: Understanding Hydrocarbon Families
Before we dive into the naming rules, let's quickly review the three main aliphatic (non-aromatic) hydrocarbon families we'll be discussing:
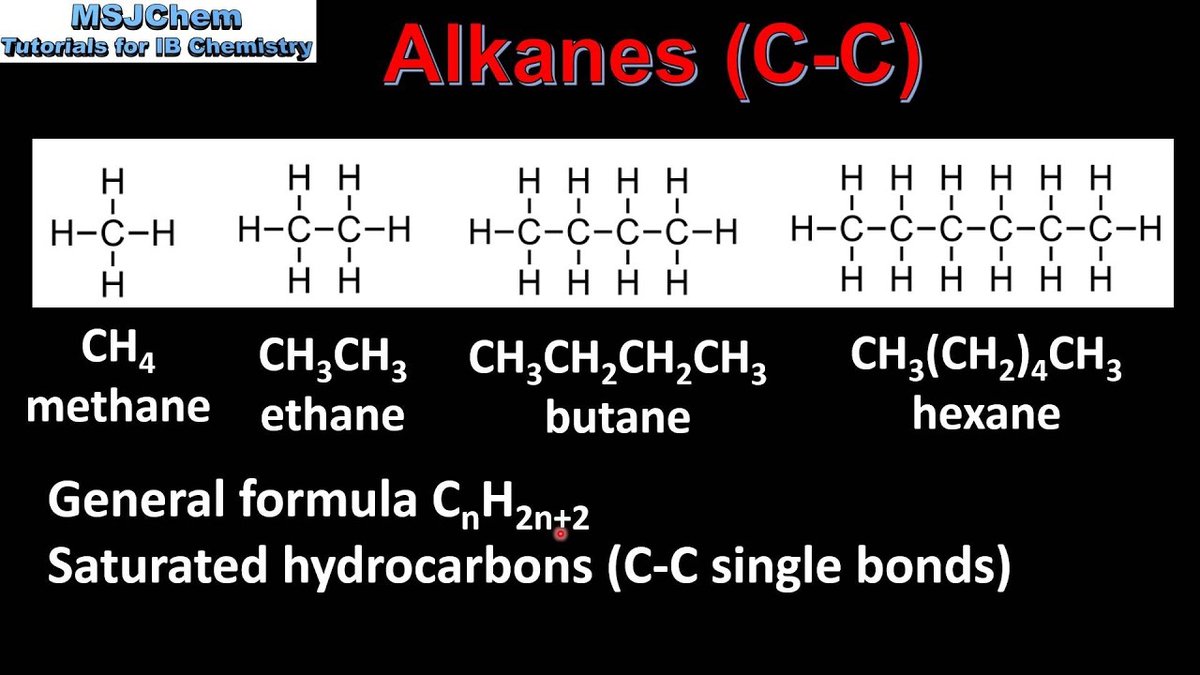
- Alkanes: These are the simplest, most saturated hydrocarbons. Every carbon atom is bonded to the maximum number of hydrogen atoms possible, and all carbon-carbon bonds are single bonds. Think of them as the stable, straightforward relatives. Their general formula is CnH2n+2. Examples: methane (CH4), ethane (CH3CH3).
- Alkenes: Introducing a bit of excitement! Alkenes contain at least one carbon-carbon double bond (C=C). This double bond makes them "unsaturated" because they have fewer hydrogen atoms than their alkane counterparts. The double bond also introduces rigidity and reactivity. Their general formula is CnH2n. Examples: ethene (CH2=CH2), propene (CH3CH=CH2).
- Alkynes: The most unsaturated of the bunch, alkynes feature at least one carbon-carbon triple bond (C≡C). These bonds are even more rigid and reactive than double bonds. Their general formula is CnH2n-2. Examples: ethyne (HC≡CH), propyne (CH3C≡CH).
Now, with our basic understanding of these families in place, let's crack the code of IUPAC naming.
Your Step-by-Step Guide to Naming Hydrocarbons
Think of IUPAC naming as a checklist. You go through each step, making decisions based on the molecule's structure, until you arrive at its unique identifier.
Step 1: Unearthing the Parent Chain (The Backbone)
This is the most critical first step: identifying the longest continuous carbon chain in your molecule. This chain forms the "parent" or "root" name.
- For Alkanes: Simply find the longest continuous sequence of carbon atoms. It doesn't have to be straight across the page; it can bend and weave!
- Once you find it, count the carbons. The number of carbons dictates the prefix:
- 1 Carbon: meth-
- 2 Carbons: eth-
- 3 Carbons: prop-
- 4 Carbons: but-
- 5 Carbons: pent-
- 6 Carbons: hex-
- 7 Carbons: hept-
- 8 Carbons: oct-
- 9 Carbons: non-
- 10 Carbons: dec-
- Add the suffix "-ane" for alkanes.
- Example: An 8-carbon alkane chain is "octane."
- For Alkenes and Alkynes: Here's where the rule gets a crucial tweak. The parent chain must be the longest continuous carbon chain that contains all the multiple bonds (double or triple). Even if there's a longer chain that doesn't include all the multiple bonds, you must choose the one that does.
- The prefix for the carbon count remains the same (eth-, prop-, etc.).
- The suffix changes to "-ene" for alkenes and "-yne" for alkynes.
- Example: If a chain has six carbons, but only a 5-carbon chain contains the double bond, the parent name will be "pentene," not "hexene."
Step 2: Numbering for Clarity (And Low Numbers!)
Once you've identified your parent chain, you need to number its carbon atoms. This isn't arbitrary; there's a system to ensure consistency.
- For Alkanes: Number the parent chain from the end that gives the substituent groups (the branches off the main chain) the lowest possible numbers.
- Example: If a methyl group is on C2 from the left end and C5 from the right end of a hexane chain, you'd number from the left, making it "2-methylhexane."
- For Alkenes and Alkynes: This rule is even more rigid. You must number the carbon atoms from the end that gives the lowest possible number to the first carbon atom of the multiple bond. This takes priority over substituent numbering.
- Example: If a double bond is between C1 and C2 from one end, and C5 and C6 from the other end of a hexene, you number from the first end. It's a "hex-1-ene" (or "1-hexene").
- Tie-breaker (Equidistant Multiple Bonds): If a multiple bond is equidistant from both ends of the chain, then you number from the end that gives the substituents the lowest possible numbers.
- Tie-breaker (Double vs. Triple Bond): If both a double and a triple bond are present and equidistant from the chain ends, the double bond takes priority for the lower number. This is a key distinction!
Step 3: Pinpointing Multiple Bond Locations
For chains of four or more carbons, you need to clearly state where the multiple bond lives.
- The position of the multiple bond is indicated by the number of its first carbon atom. This number is typically placed immediately before the suffix (e.g., hex-2-ene, pent-1-yne) or sometimes before the parent chain name (e.g., 2-hexene, 1-pentyne).
- IUPAC generally encourages placing the location identifier as close to the feature it describes as possible, so "hex-2-ene" is often preferred over "2-hexene" in modern nomenclature, though both are recognized.
- Example: If a double bond starts at the second carbon of a 6-carbon chain, it's hex-2-ene.
Step 4: Naming and Locating the Branches (Substituents)
Once the parent chain is named and numbered, it's time to identify and locate any groups branching off it. These are called substituent groups.
- Naming Substituents:
- Alkyl groups (derived from alkanes by removing one hydrogen) are the most common. Replace the "-ane" suffix with "-yl."
- CH3- (from methane): methyl
- CH3CH2- (from ethane): ethyl
- CH3CH2CH2- (from propane): propyl
- (CH3)2CH- (from propane, isopropyl): isopropyl
- Substituents containing carbon-carbon triple bonds are named alkynyl (e.g., ethynyl for HC≡C-).
- Locating Substituents: Their position is indicated by the number of the carbon atom on the parent chain to which they are attached.
- Multiple Identical Substituents: If you have multiple identical substituents (e.g., two methyl groups), use prefixes:
- di- (for two)
- tri- (for three)
- tetra- (for four)
- Example: Two methyl groups on carbons 2 and 4 would be "2,4-dimethyl."
- Alphabetical Order: When there are different types of substituents, they are listed alphabetically in the name.
- Important Note: Prefixes like di-, tri-, tetra- are ignored when alphabetizing. Isopropyl, however, is alphabetized by 'i'.
- Example: In "4-ethyl-2-methylhexane," ethyl comes before methyl.
Step 5: When Multiple Bonds Multiply (Dienes, Trienes, Enynes)
Some hydrocarbons have more than one multiple bond. IUPAC has rules for these as well.
- Multiple Double Bonds: If a compound has two double bonds, use the suffix "-diene." For three, use "-triene," and so on.
- You'll need to indicate the position of each double bond.
- Example: A 6-carbon chain with double bonds at carbons 1 and 3, and a methyl group at carbon 5: "5-methylhexa-1,3-diene." Notice the 'a' added to "hex-" before "-diene." This is common for these structures.
- Multiple Triple Bonds: Similar to double bonds, use "-diyne" for two triple bonds, "-triyne" for three, etc.
- Both Double and Triple Bonds (Enynes): If a molecule contains both double and triple bonds, the double bond's root name precedes the triple bond's root name. The suffix becomes "-en-yne."
- Remember the numbering priority: If equidistant from the ends, the double bond gets the lower number. Otherwise, the multiple bond closest to an end gets the lowest number.
- Example: A 6-carbon chain with a double bond at C1 and a triple bond at C5: "hex-1-en-5-yne."
A Special Twist for Alkenes: Geometric Isomers (cis/trans & E/Z)
Here's where alkenes reveal their unique personality. Unlike single bonds, which can rotate freely, the carbon-carbon double bond has a fixed geometry. This rigidity prevents rotation, meaning that groups attached to the double bond carbons can be oriented in different ways in space, leading to distinct molecules called geometric isomers. These aren't just minor variations; they are different substances with potentially different physical and chemical properties.
Geometric isomerism does not occur if a carbon atom in the double bond has two identical attached groups (e.g., ethene, propene, or an alkene where one C of the C=C has two methyls).
The 'cis' and 'trans' System
This older system is still widely used, especially for simpler alkenes. It focuses on the relative position of the "parent chain" across the double bond:
- 'cis' (Latin for "on the same side"): The main carbon chain enters and leaves the double bond on the same side (either both above or both below the double bond plane).
- 'trans' (Latin for "across"): The main carbon chain enters and leaves the double bond on opposite sides of the double bond.
- Example: cis-2-butene vs. trans-2-butene.
The IUPAC 'E' and 'Z' System
While 'cis' and 'trans' work well for many cases, they can be ambiguous when more than two different groups are attached to the double bond carbons. The IUPAC 'E' and 'Z' system provides an unambiguous way to designate geometric isomers for any alkene. It's based on assigning priorities to the groups attached to each carbon of the double bond (using Cahn-Ingold-Prelog priority rules, which involve atomic number).
- Assign Priority: For each carbon atom involved in the double bond, rank the two groups attached to it as either "high priority" or "low priority." (Higher atomic number gets higher priority).
- Compare Sides:
- 'Z' (zusammen - German for "together"): The two higher-priority groups are on the same side of the double bond.
- 'E' (entgegen - German for "opposite"): The two higher-priority groups are on opposite sides of the double bond.
It's important to note that E/Z isomers often correspond to trans/cis, respectively, but not always. The E/Z system is more robust and universally applicable. Designating 'E' or 'Z' is crucial for identifying specific substances, particularly in fields like pharmacology, where the shape of a molecule dictates its biological activity.
Rings in the Mix: Cyclic Hydrocarbons
What happens when our hydrocarbon chain curls back on itself and forms a ring? We add the prefix "cyclo-."
- Cycloalkanes: Single bonds only. Example: Cyclohexane.
- Cycloalkenes: Contain a double bond within the ring. The double bond carbons are always considered C1 and C2, but you generally don't need to specify their position (e.g., cyclohexene, not cyclohex-1-ene). Numbering then proceeds to give substituents the lowest possible numbers.
- Example: Cyclohexene, cyclooctene.
Putting It All Together: Worked Examples
Let's apply these rules to a few examples to solidify your understanding.
Example 1: 4-methyl-2-pentene
- Parent Chain: The suffix "-pentene" tells us it's a 5-carbon chain with a double bond. "2-pentene" means the double bond is between C2 and C3.
- Substituents: "4-methyl" means a methyl group (CH3) is attached to the fourth carbon of the parent chain.
- Drawing It:
- Start with a 5-carbon chain.
- Place the double bond between C2 and C3.
- Add a methyl group to C4.
- Fill in remaining hydrogens.
CH3
|
CH3-CH=CH-CH-CH3
^ ^ ^
C2-C3 C4
Example 2: 2-ethyl-1-butene
- Parent Chain: "1-butene" indicates a 4-carbon chain with a double bond starting at C1 (i.e., between C1 and C2).
- Substituents: "2-ethyl" means an ethyl group (CH3CH2) is attached to the second carbon of the parent chain.
- Drawing It:
- Draw a 4-carbon chain.
- Place the double bond between C1 and C2.
- Add an ethyl group to C2.
- Fill in hydrogens.
CH2CH3
|
CH2=C-CH2CH3
^
C2 - Self-correction check: Is 2-ethyl-1-butene actually the longest chain? If we go from the ethyl group, through C2, and down to C4, we get a 5-carbon chain: CH3CH2-C(CH2)=CH2-CH3.
- The double bond is between C2 and C3 of this new longer chain (counting from the end of the ethyl group).
- This would make it 2-methyl-1-pentene. Ah! This is a classic trick. The parent chain must be the longest chain containing the double bond. In 2-ethyl-1-butene, if you count the carbons: CH2=C(CH2CH3)-CH2-CH3, the longest chain containing the double bond is the 4-carbon 'butene' chain. The ethyl group is indeed a substituent. My initial drawing shows the ethyl group on C2 correctly, but the resulting molecule's name implies the longest chain including the double bond is 4 carbons.
Let's re-draw 2-ethyl-1-butene to be absolutely clear:
CH2CH3
|
CH2 = C - CH2 - CH3
1 2 3 4
The double bond is between C1 and C2. The ethyl group is on C2. The longest continuous chain containing the double bond is indeed 4 carbons, named "1-butene". So the name is correct as 2-ethyl-1-butene.
Example 3: E-3-Methylhex-2-ene
- Parent Chain: "hex-2-ene" means a 6-carbon chain with a double bond between C2 and C3.
- Substituents: "3-methyl" means a methyl group (CH3) is attached to C3.
- Geometric Isomerism: "E-" tells us this is the entgegen (opposite) isomer. We need to apply priority rules.
- Double bond carbons: C2 and C3.
- Groups on C2: H (low priority), CH3 (high priority, relative to H).
- Groups on C3: CH3 (low priority, relative to CH2CH2CH3), CH2CH2CH3 (propyl group, high priority).
- For 'E' isomer: The high priority groups on C2 (CH3) and C3 (propyl) must be on opposite sides of the double bond.
- Drawing It:
CH3
/
CH3-C = C-CH2CH2CH3
|
H CH3
(This shows CH3 on C2 and CH3 on C3 on the same side, which would be Z. Let's adjust for E.)
High priority on C2: CH3
High priority on C3: CH2CH2CH3
CH3
/
CH3-C = C-H
|
H CH2CH2CH3
(No, this is wrong. The methyl is on C3, not C2. Let's redraw with correct numbering and substituent)
Parent chain: Hex-2-ene. C2=C3. Methyl on C3.
C1 C2 C3 C4 C5 C6
CH3-CH=C-CH2CH2CH3
|
CH3
Now let's apply E/Z to the C2=C3 double bond.
Groups on C2: H (low), CH3 (high, from C1).
Groups on C3: CH3 (high, the substituent), CH2CH2CH3 (high, the rest of the chain from C4). No, this is where the Cahn-Ingold-Prelog (CIP) rules are critical.
Groups on C3: Methyl (CH3) and Propyl (CH2CH2CH3). Propyl (C-C-C) has higher priority than Methyl (C).
So, for C2: H (low), C1-CH3 (high).
For C3: C3-CH3 (low, because C-C, vs. C-C-C), C4-CH2CH2CH3 (high).
To be 'E', the high-priority groups on C2 (C1-CH3) and C3 (C4-CH2CH2CH3) must be on opposite sides.
CH3 (from C1, HIGH)
/
C2 = C3
|
H CH2CH2CH3 (from C4, HIGH)
CH3 (from C3 substituent, LOW)
This is the E-isomer. The methyl group on C3 is pointing down, and the propyl group from C4 is pointing up, while the methyl on C2 is up, and H on C2 is down. The two high priority groups (methyl from C1, propyl from C4) are trans to each other across the double bond.
Correct diagram for E-3-Methylhex-2-ene:
CH3
|
CH3 - C = C - CH2CH2CH3
/
H CH3 (the 3-methyl group)
Wait, no, the drawing and explanation need to align precisely with E/Z. Let's restart the drawing based on the high priority definition.
C2 Groups:
- -CH3 (from C1): Priority 1
- -H: Priority 2
C3 Groups: - -CH2CH2CH3 (from C4, C5, C6): Priority 1
- -CH3 (the 3-methyl substituent): Priority 2
For 'E' (entgegen - opposite), the two priority 1 groups must be on opposite sides of the double bond.
CH3 (P1 on C2)
|
CH3-C = C-H
/
H CH2CH2CH3 (P1 on C3)
|
CH3 (P2 on C3) <- This is wrong, the methyl group is attached directly to C3.
Let's make sure the groups on C3 are clear.
C3 has a methyl substituent and is connected to C4 of the main chain.
So, groups on C3 are: - -CH3 (the methyl substituent)
- -CH2CH2CH3 (the continuation of the main chain: C4-C5-C6)
Priority on C3:
-CH2CH2CH3 (P1, as C-C-C branch is higher priority than C branch)
-CH3 (P2)
So, for E-3-Methylhex-2-ene:
C2 has P1=CH3, P2=H
C3 has P1=CH2CH2CH3, P2=CH3
E-isomer means P1 on C2 and P1 on C3 are on opposite sides.
CH3 (P1 on C2)
|
CH3 - C = C - H (P2 on C2)
/
H CH2CH2CH3 (P1 on C3)
|
CH3 (P2 on C3)
This arrangement correctly shows the highest priority groups (CH3 from C1 on C2, and the propyl chain from C4 on C3) on opposite sides of the double bond. The methyl substituent is connected to C3 and points opposite to the main chain continuation.
Why Precision in Naming Matters (Beyond the Classroom)
The systematic naming of hydrocarbons isn't just an academic exercise. Its real-world implications are vast and critical:
- Pharmaceuticals: The specific spatial arrangement of atoms can drastically change a drug's efficacy and side effects. For instance, an (E)-isomer of a drug might be therapeutic, while its (Z)-isomer could be toxic or inactive. Precise IUPAC naming, including E/Z designations, ensures that researchers and manufacturers are always talking about the exact same molecule.
- Industrial Chemistry: In synthesizing polymers, fuels, or other organic compounds, identifying reactants and products correctly is paramount for process control, safety, and yield optimization. Misnaming a feedstock could lead to disastrous results.
- Environmental Science: When studying pollutants or natural compounds, their exact structure affects their degradation pathways, toxicity, and interaction with biological systems. Accurate nomenclature facilitates clear communication among scientists globally.
- Research and Development: Every new compound discovered or synthesized needs an unambiguous name. This allows scientists worldwide to reproduce experiments, build upon previous work, and advance chemical knowledge.
In essence, IUPAC nomenclature is the bedrock of chemical communication. Without it, the modern world, built on sophisticated chemical processes and compounds, would simply grind to a halt. If you ever need a quick check or want to explore complex structures, a reliable IUPAC name generator can be an invaluable tool for validating your manual naming efforts.
Common Pitfalls and Pro Tips
- Don't force the parent chain to be straight. It can bend! Always trace every possible continuous carbon path to find the truly longest one.
- Multiple bonds are king! For alkenes and alkynes, the multiple bond's location dictates numbering priority, even if it means substituents get higher numbers.
- Alphabetize carefully. Remember that di-, tri-, tetra- are ignored for alphabetization, but iso- is usually included.
- Check for isomerism. If you have an alkene, always consider if 'cis/trans' or 'E/Z' applies. It's a distinct structural feature that must be named.
- Practice, practice, practice! The best way to master IUPAC naming is to work through dozens of examples, both drawing structures from names and naming structures from drawings.
Beyond the Basics: Your Next Steps
You've now got a solid foundation for naming alkanes, alkenes, and alkynes according to IUPAC rules. But organic chemistry is a vast and fascinating field. From here, you might explore:
- Functional Groups: How do -OH (alcohols), -COOH (carboxylic acids), and other groups change naming priorities and properties?
- Stereochemistry: Delve deeper into molecules with chirality and optical isomerism (R/S nomenclature).
- Aromatic Compounds: Learn about benzene rings and their unique naming conventions.
- Cyclic Compounds: Expand your understanding of rings, including polycyclic systems and bicyclic compounds.
Mastering hydrocarbon nomenclature is more than just passing a test; it's about gaining a language that unlocks a deeper understanding of the molecular world around us. Keep exploring, keep practicing, and you'll find the intricate logic of organic chemistry incredibly rewarding.