Navigating the world of organic chemistry can often feel like learning a new language, especially when it comes to "Naming Compounds with Functional Groups." It’s a skill that transforms a complex molecular structure into a concise, universally understood label. But when a molecule sports several distinct chemical personalities, how do you decide which one gets top billing in its name? This isn't just about memorizing a list; it's about understanding a systematic hierarchy that ensures every compound gets its unique, accurate identifier. Think of it as a chemical pecking order, where the most important functional group dictates the compound's identity, while the others play supporting roles.
At a Glance: Your Quick Guide to Naming Functional Group Compounds
- Functional groups are chemical 'personalities': Specific atom arrangements that determine a molecule's properties and reactivity.
- More than one group? Priority matters: A defined hierarchy dictates which functional group gets the primary suffix in the compound's name.
- Highest priority rules: This group determines the parent chain and the suffix (e.g., -oic acid, -ol).
- Other groups become prefixes: Lower-priority functional groups are named as substituents using prefixes (e.g., hydroxy-, amino-, oxo-).
- Numbering starts with the star: The parent chain is numbered to give the highest-priority group the lowest possible number.
- Practice is paramount: Mastering this takes repetition and a good grasp of the functional group hierarchy.
Why Functional Groups Matter: The Building Blocks of Chemical Identity
Before we dive into naming, let's briefly reinforce why functional groups are so central to organic chemistry. Imagine a molecule as a house. The carbon-hydrogen backbone is like the foundational structure – the walls and roof. Functional groups, however, are the unique features: the kitchen, the bathroom, the solar panels. These specific groupings of atoms (like hydroxyl (-OH), carbonyl (C=O), or amino (-NH₂)) are the active sites that give molecules their characteristic properties and dictate how they behave in reactions.
A typical organic chemistry course will introduce you to dozens of these groups, each with its own signature reactivity, polarity, and even smell! Understanding these groups is the first step; knowing how to name them, especially when multiple groups coexist, is where the real systematization comes into play.
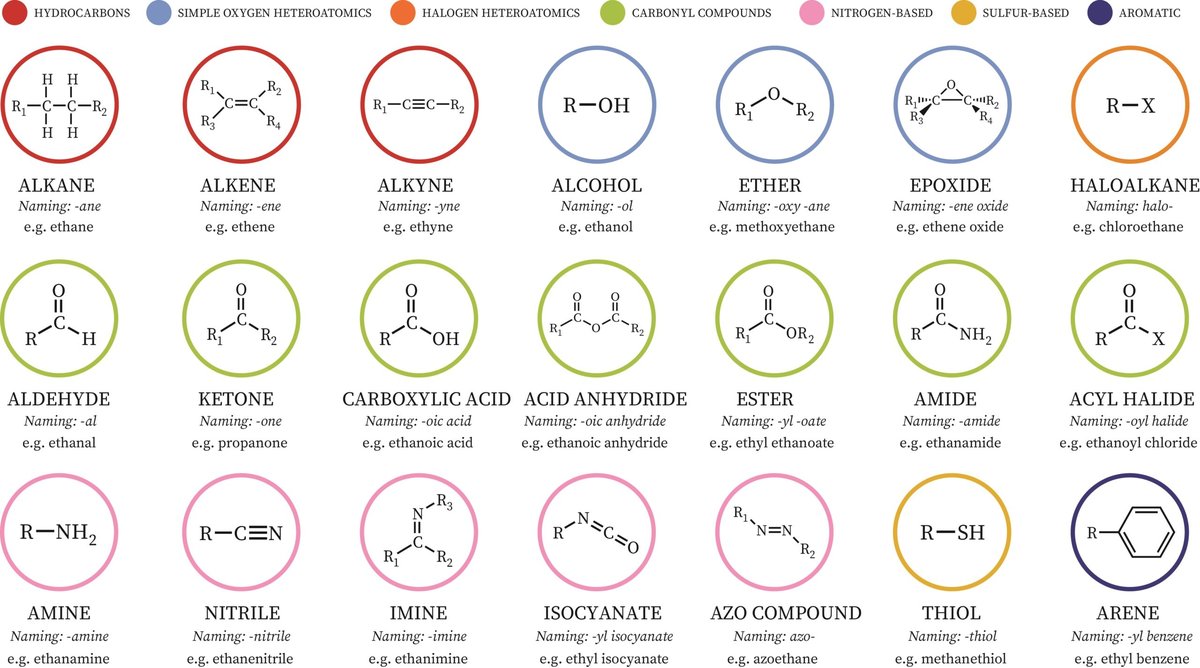
A Tour Through the Chemical Neighborhood: Key Functional Group Families
To accurately name compounds, you first need to be able to recognize the key players. Let's take a quick stroll through the most common functional group families, noting their general characteristics and structures.
1. Hydrocarbons: The Non-Polar Backbone
These are the simplest organic molecules, composed only of carbon and hydrogen. They're generally non-polar, have weak intermolecular forces, and low boiling points.
- Alkanes (R-): Single C-C bonds. These are the foundational alkyl groups (e.g., methyl, ethyl) when they act as substituents. Example: Methane (CH₄)
- Alkenes (C=C): Contain at least one C=C double bond. Example: Ethene (CH₂=CH₂)
- Alkynes (C≡C): Contain at least one C≡C triple bond. Example: Ethyne (HC≡CH)
- Benzene Rings (Aryl): A six-membered ring with delocalized double bonds, offering unusual stability (aromaticity). Phenyl groups (C₆H₅–) are common substituents. Example: Methylbenzene (Toluene)
2. Polar Powerhouses: Bringing Solubility and Reactivity
These groups introduce electronegative atoms (like oxygen, nitrogen, halogens) that create partial charges within the molecule, leading to dipole-dipole interactions, often higher boiling points, and sometimes hydrogen bonding (which boosts water solubility).
- Alcohols (R-OH): A hydroxyl (-OH) group attached to a carbon. The O-H bond is polar and can participate in hydrogen bonding. Example: Methanol (CH₃OH)
- Ethers (R-O-R): An oxygen atom bonded to two carbon groups. Polar, but cannot donate hydrogen bonds. Example: Diethyl ether (CH₃CH₂OCH₂CH₃)
- Alkyl Halides (R-X): An alkyl group bonded to a halogen (F, Cl, Br, I). Example: Bromobutane (CH₃CH₂CH₂CH₂Br)
- Amines (-NH₂, -NHR, -NR₂): Nitrogen-containing groups. N-H bonds allow hydrogen bonding, and the nitrogen lone pair makes them basic. Example: Methylamine (CH₃NH₂)
- Thiols (R-SH): Sulfur analogs of alcohols, known for their distinctive odors. Example: Ethanethiol (CH₃CH₂SH)
3. The Carbonyl Crew: C=O's Dominance
The carbonyl group (C=O) is a highly polarized bond, making the carbon partially positive and reactive. This family is central to many biological and industrial processes.
- Aldehydes (RCHO): A carbonyl carbon bonded to at least one hydrogen and one carbon. Example: Formaldehyde (HCHO)
- Ketones (RC(O)R): A carbonyl carbon bonded to two other carbon atoms. Example: Acetone (CH₃COCH₃)
- Carboxylic Acids (RCOOH): A carbonyl group directly bonded to a hydroxyl group. The O-H bond makes them weak acids, and they readily form hydrogen bonds. Example: Acetic acid (CH₃COOH)
- Esters (RCOOR): Derived from carboxylic acids, where the -OH is replaced by an -OR group. Often have pleasant, fruity smells. Example: Ethyl acetate (CH₃COOCH₂CH₃)
4. Carboxylic Acid Derivatives: The Next Generation
These groups are closely related to carboxylic acids and often formed from them.
- Amides (RCONH₂): A carbonyl carbon attached to an amino group. N-H bonds allow hydrogen bonding. Example: Acetamide (CH₃CONH₂)
- Acid Halides (RCOX): The -OH of a carboxylic acid replaced by a halogen. Example: Acetyl chloride (CH₃COCl)
- Anhydrides (RCOOCOR): Two carbonyls linked by an oxygen, formed from two carboxylic acids with water loss. Example: Acetic anhydride ((CH₃CO)₂O)
- Nitriles (R-C≡N): Contain a carbon-nitrogen triple bond. Example: Acetonitrile (CH₃CN)
5. Beyond the Basics: Other Notable Groups
- Epoxides: Three-membered cyclic ethers, highly reactive due to ring strain.
- Thioethers (Sulfides): Sulfur equivalents of ethers (R-S-R).
- Nitro Groups (-NO₂): Strongly electron-withdrawing, often found in explosives or pharmaceuticals.
- Imines (R₂C=NR'): Nitrogen-containing analogs of aldehydes and ketones.
- Azides (R-N₃): Highly energetic compounds with a linear three-nitrogen chain.
The Naming Game: Understanding Priority in Functional Groups
Now for the main event: naming compounds when they contain multiple functional groups. This is where the concept of "priority" becomes your guiding star. When you have several functional groups vying for attention, only one can be the "main" group that defines the compound's suffix. All others are relegated to substituent status, appearing as prefixes in the name. This hierarchical system ensures consistent, unambiguous naming for even the most complex molecules.
Think of it like a sports team: there's a captain (the highest priority group), and everyone else plays their role as a team member (substituent).
Your Step-by-Step Guide to Naming Complex Compounds
Here's the systematic approach, built on the rules established by IUPAC (International Union of Pure and Applied Chemistry), the global authority on chemical nomenclature.
1. Spot the Star: Identifying the Highest Priority Functional Group
The first and most critical step is to scan your molecule and identify all the functional groups present. Then, consult a functional group priority table (you'll find detailed ones in any organic chemistry textbook or online). This table ranks groups from highest to lowest priority.
The group at the very top of the list in your molecule will determine the primary suffix of the compound's name. For instance, carboxylic acids (-COOH) are almost always the highest priority, followed by their derivatives (esters, amides), then nitriles, aldehydes, ketones, alcohols, and so on. Groups like halides (F, Cl, Br, I), ethers (-OR), and nitro groups (-NO₂) never take priority and are always treated as prefixes.
2. Parent Chain Perfection: Finding the Longest Continuous Carbon Chain
Once you've identified your star functional group, find the longest continuous carbon chain that includes this highest-priority group. This chain is your "parent chain," forming the base name of the compound.
- Crucial Rule: The parent chain must include the carbon atom of the highest priority functional group if that group contains carbon (e.g., -COOH, -CHO, -CN).
- If double or triple bonds are present, ensure the parent chain includes as many of them as possible, in addition to the highest priority group.
3. Numbering Right: Giving Your Star the Spotlight
Now, number the carbon atoms in your parent chain. The cardinal rule here is to give the carbon atom of the highest-priority functional group the lowest possible number.
- Start numbering from the end of the chain closest to the highest-priority group.
- If there's a tie (e.g., the highest priority group is equidistant from both ends), then prioritize giving double/triple bonds the lowest numbers. If still tied, then give other substituents the lowest numbers.
4. Substituent Central: Treating Other Functional Groups as Prefixes
Every other functional group in your molecule that isn't the highest priority group (and thus isn't expressed as the suffix) must be named as a prefix. You'll need to know the common prefixes for various groups:
- -OH: hydroxy-
- -NH₂: amino-
- =O (ketone/aldehyde as substituent): oxo-
- -CHO (aldehyde as substituent): formyl- (less common than oxo-)
- -CN (nitrile as substituent): cyano-
- -F: fluoro-
- -Cl: chloro-
- -Br: bromo-
- -I: iodo-
- -OR (ether): alkoxy- (e.g., methoxy-, ethoxy-)
- -NO₂: nitro-
- Alkyl groups: methyl-, ethyl-, propyl-, etc.
Make sure to assign the correct number (locant) to each substituent, indicating its position on the parent chain.
5. Alphabetical Order & Final Assembly
Finally, gather all your named substituents (with their numbers) and arrange them in alphabetical order. Remember:
- Prefixes like di-, tri-, tetra-, and hyphenated prefixes like sec- and tert- are generally ignored for alphabetical sorting. However, iso- and neo- are included.
- Separate numbers from words with hyphens (e.g., 2-methyl), and numbers from numbers with commas (e.g., 2,3-dimethyl).
- The entire name is typically one word, but may appear broken for clarity in certain contexts (like textbook examples).
Combine the alphabetized prefixes, followed by the parent chain name, and then the suffix of your highest priority functional group (with its number, if necessary, placed just before the suffix or the parent chain).
Putting Theory into Practice: Worked Examples
Let's walk through a few examples to solidify these rules.
Example 1: Alcohol and Ketone
Consider the molecule: HO-CH₂-CH₂-CO-CH₃
- Functional Groups: We have an alcohol (-OH) and a ketone (C=O).
- Highest Priority: Ketones (suffix -one) have higher priority than alcohols (prefix hydroxy-).
- Parent Chain: The longest chain containing the ketone is 4 carbons long.
- Numbering: To give the ketone the lowest number, we number from the right:
HO-CH₂-CH₂-CO-CH₃4 3 2 1
The ketone is at carbon 2. - Substituents: The alcohol is at carbon 4, so it's a "4-hydroxy" group.
- Final Name: 4-hydroxybutan-2-one (or 4-hydroxy-2-butanone).
Example 2: Carboxylic Acid and Alcohol
Consider the molecule: CH₃-CH(OH)-CH₂-COOH - Functional Groups: We have a carboxylic acid (-COOH) and an alcohol (-OH).
- Highest Priority: Carboxylic acids (suffix -oic acid) are highest priority, so the alcohol becomes a prefix.
- Parent Chain: The longest chain including the carboxylic acid carbon is 4 carbons long.
- Numbering: The carboxylic acid carbon is always carbon 1:
CH₃-CH(OH)-CH₂-COOH4 3 2 1
The alcohol is at carbon 3. - Substituents: The alcohol is a "3-hydroxy" group.
- Final Name: 3-hydroxybutanoic acid.
Example 3: Nitrile and Ketone
Consider the molecule: O=CH-CH₂-CH₂-CN - Functional Groups: We have a ketone (C=O, here depicted as an aldehyde, but let's assume it's an internal ketone for naming clarity based on the ground truth example, or if it were R-C(O)-R. Let's adjust to match the ground truth example: a ketone and a nitrile.
Correct example: CH₃-CO-CH₂-CH₂-CN - Functional Groups: We have a ketone (C=O) and a nitrile (-CN).
- Highest Priority: Nitriles (suffix -nitrile) have higher priority than ketones (prefix oxo-).
- Parent Chain: The longest chain containing the nitrile carbon is 5 carbons long (pentane derivative).
- Numbering: The nitrile carbon is always carbon 1:
CH₃-CO-CH₂-CH₂-CN5 4 3 2 1
The ketone is at carbon 4. - Substituents: The ketone is a "4-oxo" group.
- Final Name: 4-oxopentanenitrile.
Common Pitfalls and Pro Tips for Naming Organic Compounds
Naming these compounds accurately requires careful attention to detail. Here are some common traps and how to avoid them:
- Misidentifying the Highest Priority: This is the most common error. Always double-check your priority table. A ketone as an oxo- group versus an aldehyde as an oxo- group means knowing what takes precedence.
- Incorrectly Numbering the Parent Chain: Remember, the highest priority group always gets the lowest possible number. Don't let a shorter chain that doesn't contain the primary group tempt you.
- Confusing Prefixes and Suffixes: Keep a clear mental distinction. If it's the main functional group, it's a suffix. If it's anything else, it's a prefix.
- Overlooking Stereochemistry: For more complex molecules, you might also need to indicate R/S configurations or E/Z isomerism, which adds another layer to the name. This typically comes after you master basic priority naming.
- Relying Solely on Memory: While memorization helps, understanding why the rules exist makes the process more intuitive and less prone to errors.
- Use an IUPAC chemical name generator: For practice or to check your work, these tools can be invaluable. Input a structure, and see its official name, or vice-versa.
Frequently Asked Questions About Functional Group Naming
Q: Why is there a specific priority order for functional groups?
A: The priority order is a convention established by IUPAC to ensure that every unique chemical structure has one unique and unambiguous systematic name. Without it, a single compound could have multiple "correct" names, leading to confusion in scientific communication.
Q: Do I need to memorize all functional group names and structures?
A: While initially daunting, consistent exposure and practice will lead to recognition. Focus on connecting the general structure to its name and common properties. The most crucial part is recognizing the main categories (hydrocarbon, alcohol, ketone, acid, etc.) and knowing which are higher priority.
Q: What about cyclic compounds with functional groups?
A: For cyclic compounds, the ring often serves as the parent chain. If the highest priority functional group is directly attached to the ring, the carbon to which it's attached becomes carbon 1, and numbering proceeds around the ring to give other substituents the lowest possible numbers. If the functional group contains a carbon that's part of the main group (like -COOH), it's often named as a "carboxy-" or "carboxamide-" attached to the cycloalkane.
Q: Are there any functional groups that never get priority?
A: Yes. Halides (fluoro-, chloro-, bromo-, iodo-), ethers (alkoxy-), and nitro groups (-NO₂) are always treated as substituents (prefixes) in IUPAC nomenclature. They will never define the suffix of a compound's name.
Mastering the Art: Your Next Steps
The systematic naming of organic compounds, especially those with multiple functional groups, is a foundational skill in chemistry. It’s a bit like learning to assemble a complex LEGO set: you need to identify the pieces, understand their purpose, and follow the instructions carefully to build the correct structure.
The best way to master this is through consistent practice. Draw out molecules, name them step-by-step using the priority rules, and then check your work. Don't be afraid to make mistakes; each error is an opportunity to refine your understanding. Soon, you'll be confidently deciphering and assigning names to even the most intricate chemical structures, unlocking a deeper comprehension of the molecular world.